"Capturing aerial insects available as food for chimney swifts in western Pennsylvania using a tethered balloon"
Published in the Journal of the Pennsylvania Academy of Science
Spring 2021
94(1-2):103-117 (reprinted here with permission)

Sutton Hall
Balloon Methodist Old
Church Courthouse
Orange research balloon flying 200 feet over Indiana, Pennsylvania
(Photo taken from the Catholic Church in the Northeast)
|
LINK TO PDF VERSION Print "Letter," "Fit to Paper," "Two-sided." |
Photos |
| Photos of Assistants | List of Assistants |
Authors
Abstract: This study presents the relative abundance of aerial insects available for chimney swifts (Chaetura pelagica) to eat in Indiana, Pennsylvania in April, May, and June of 2006. Aerial insects were captured on acrylic “sticky traps” attached to the tether rope of a 3.048-m-diameter helium balloon at heights of 27 to 55 m above the ground. The methodology is evaluated, and we conclude that the described method can be carried out successfully and does provide useful data, but also has practical difficulties. The total sample size was 716 individual organisms (685 insects and 31 spiders) captured over 115.84 collecting hours. This study provides baseline information and recommendations for future studies within the context of conservation, especially concerning changes in relative abundance of aerial insect orders used for food by insectivores, such as chimney swifts. Historical and current aerial insect sampling methods are discussed.
Keywords: aerial insects, chimney swifts, Chaetura pelagica, insectivore, tethered balloon
![]()
Journal of the Pennsylvania Academy of Science, Vol. 94, Nos. 1–2, 2020
Copyright © 2021 The Pennsylvania State University, University Park, PA
Accepted for publication 31 January 2021
DOI: 10.5325/jpennacadscie.94.1-2.0103
![]()
The chimney swift (Chaetura pelagica) is an aerial insectivore
commonly seen during the day in the spring and summer months as it flies
over urban areas in Pennsylvania while “hawking” aerial insects, its almost
exclusive source of food (Zammuto, Franks, and Preston 1981; Wilson,
Brauning, and Mulvihill 2012). The current population decrease in chimney swifts is linked to the current
decrease in flying insects, thought to be due to such factors as pesticides,
non-native plant species, and climate change (Nebel et al. 2010; Nocera et al.
2012; Pennsylvania Game Commission and Pennsylvania Fish and Boat Commission [PGC-PFBC]
2015, Appendix 1.4—Birds; Michel et al. 2016; Hallmann et al. 2017; Cox et al.
2019; Adler 2020; Tallamy and Shriver 2021; Wagner et al. 2021). In the past,
reduced availability of nesting sites for chimney swifts was thought to be as,
or more, detrimental than aerial insect declines (COSEWIC 2007). However, more
recent studies suggest that declines in aerial insect populations, rather than
nesting site availability, account for the decline of chimney swifts (Fitzgerald
et al. 2014; COSEWIC 2018).
According to the North American Breeding Bird Survey, the chimney swift
population overall decreased 2.61% per year for the years 1966–2017, while, in
Pennsylvania, the decline was slightly greater after 2005 at 2.66% per year
(Sauer et al. 2017). Furthermore, a 27% decline in chimney swifts occurred
between the first Atlas of Breeding Birds in Pennsylvania (Brauning 1992)
and the Second Atlas of Breeding Birds in Pennsylvania (Wilson, Brauning, and
Mulvihill 2012) 20 years later.
Consequently, the chimney swift has been labeled as a “Species of Greatest
Conservation Need” in the 2015-2025 Pennsylvania Wildlife Action Plan, and this
state document emphasizes that chimney swifts are threatened by a “reduced
insect food supply caused by pesticide use” (PGC-PFBC 2015, Appendix 1.4—Birds).
Likewise, in 2018, the International Union for the Conservation of Nature and
Natural Resources (IUCN) changed the status of the chimney swift to “Vulnerable”
(BirdLife International 2018).
Therefore, it is imperative that the driving factor for chimney swift decline,
the change in aerial insect population dynamics, be conscientiously studied and
monitored to better understand the decline and to prepare possible conservation
approaches.
Insect collecting nets (sweep nets and aerial nets) are often used to capture
insects in ground vegetation and to capture insects that fly close to the ground
(Ferro 2011). However, chimney swifts and other high-flying insectivores catch
their prey much higher above the ground. Collecting techniques higher in the air
are more appropriate to better access and assess what aerial insects are
available to them as food.
This study addresses the question “What are the aerial insects available for
local chimney swifts to eat in Indiana, PA, a small town in western
Pennsylvania?” Our objectives were twofold: to explore the feasibility of
sampling aerial insects by attaching collecting panels, that is, “sticky traps”
or “flight intercept traps,” at different heights to the tether rope of a large
helium balloon, and to collect baseline data for the relative abundance of
aerial insects available in this area for chimney swifts.
MATERIALS AND METHODS
Approval was obtained from both the manager of the local Indiana County Jimmy Stewart Airport near Indiana, Pennsylvania, and the regional Federal Aviation Administration Principal Operations Inspector of the Allegheny Flight Standards District Office in Pittsburgh, PA, to fly a large, tethered, helium balloon during the day up to 60.96 m (200 ft) off the ground, the maximum allowed, from the roof of Weyandt Hall on the campus of Indiana University of Pennsylvania (IUP). The tether for the balloon was securely bolted 14.10 m (46.25 ft) above the ground to the floor of the elevated deck on the roof of Weyandt Hall. The elevation of Weyandt Hall at ground level is estimated to be 392 m (FreeMapTools 2020).
Five acrylic panels were coated on both sides with Tangle-Trap Insect Trap Coating, produced by the Tanglefoot Company, Grand Rapids, MI, and attached to the tether rope of a 3.048-m-diameter (10-ft-diameter) helium balloon, obtained from Third Dimension Advertising, Irvine, California. Tangle-Trap Insect Trap Coating was used as the sticky substance, since it is pesticide-free, clear, and odorless and able to endure all weather cycles and temperature changes. Panels were attached 27 m (88.58 ft), 34 m, 41 m, 48 m, and 55 m (180.45 ft) above the ground. Chimney swifts were often observed at these heights.
Equidistant placement of the five collecting panels was determined by the space available on the tether rope from the roof to the balloon, allowing some space (2.91 m) between the highest panel and the balloon—and some space (12.9 m) at the bottom of the tether rope above the roof. Each panel had effective dimensions of 31.62 × 31.62 cm, that is, 1000 cm² on each side. Each panel functioned as a sticky trap that captured insects as they either hit or landed on the panel surface. The total surface area for both sides of the five collecting panels was 10,000 cm², that is, one square meter.
The mechanism for the attachment and removal of the panels from the tether rope was designed by George Carenzo, science shop technician at IUP. Two bolts were used to attach each panel to two heavy metal clamps that were permanently attached at the appropriate height on the tether rope. The bolts were inserted through the clamps and the two previously drilled holes through one side of each panel and secured with wing nuts. The attachment area on the panel was next to, but not a part of, the measured sticky part of the panel. The panels were held away from the tether rope by the tension on the rope caused by the balloon. The panels were attached and detached in sequence as the balloon was deployed and recovered.
The first sampling day was April 19, 2006, two days earlier than the arrival of the chimney swifts, and the last sampling day was June 30, 2006. Chimney swifts reside primarily in urban areas in their large breeding area across most of eastern North America, and, in Pennsylvania, the breeding season starts soon after birds arrive in spring in every county in the state (Wilson et al. 2012). The arrival of chimney swifts in western Pennsylvania occurs “the third or fourth week in April” (Todd 1940), and this was the justification for the sampling dates chosen.
Even though the original research plans called for collecting data on three days each week during the months of April, May, and June, this plan proved not to be possible because of practical weather restrictions. Wind speed was a major limiting factor in being able to deploy and retrieve the balloon. Consequently, only 15 days were suitable to collect samples during April, May, and June. Five daily samples were obtained in April with a total of 36.75 sampling hours, one sample in May with a total of 8.25 sampling hours, and nine samples in June with a total of 70.84 sampling hours. Sampling times and durations varied with the day since higher wind speeds necessitated either delaying the deployment of the balloon or bringing it down earlier than originally planned.
As the balloon was retrieved, each panel was removed from the tether and put into a specially designed container that secured and separated each panel. Individual insects were carefully removed from the Tangle-Trap Insect Trap Coating on each panel and put into a jar labeled for that day as to panel height. The specimens were initially put into Histo-Clear solvent to obtain clean, undamaged specimens. After the Tangle-Trap adhesive was removed by the Histo-Clear, the specimens were preserved in 75% alcohol for identification by Tim Tomon, an entomologist at the Carnegie Museum of Natural History in Pittsburgh, PA.
More helium was continually added to the balloon due to helium leaking out through the balloon material. Amounts of helium added varied with the time between deployments of the balloon and wind speed, which accelerated this loss. The balloon was carefully moored and secured on the roof between flights. Before most deployments of the balloon, the balloon was refilled with helium.
Statistical comparisons reported in the Results include an independent samples t-test, one-way χ2 tests, and χ2 tests of independence. Graphical results are presented using percentages of individuals, while the χ2 statistical tests were conducted with the raw frequency data, the number of individuals captured.
RESULTS
The total number of individual organisms captured was 716 (685 insects and 31 spiders) over a total of 115.84 collecting hours.
The average capture rate was 6.18 captures per m² per hour of sampling time during the study period. (The number of captures for each hour is not available; however, daily counts are available.) In April, an overall average of 5.12 captures were made per hour per m². Taking each sampling day as a replicate sample results in five sampling-day averages in April with an average of 5.34 captures per hour per m² with a standard error of 1.96. In May, an average of 7.27 captures were made per hour per m² for the one sampling day. In June, an overall average of 6.61 captures were made per hour per m². In June, each sampling day was considered a replicate sample, resulting in nine sampling-day averages with an average of 7.38 captures per hour per m² with a standard error of 1.37. Because of the high variability among sampling days, no significant difference was found in the mean number of captures per hour between April and June (independent samples t-test, IV Levels = 2 months, n = 14 days, df = 12, t = -0.87, p = 0.40).
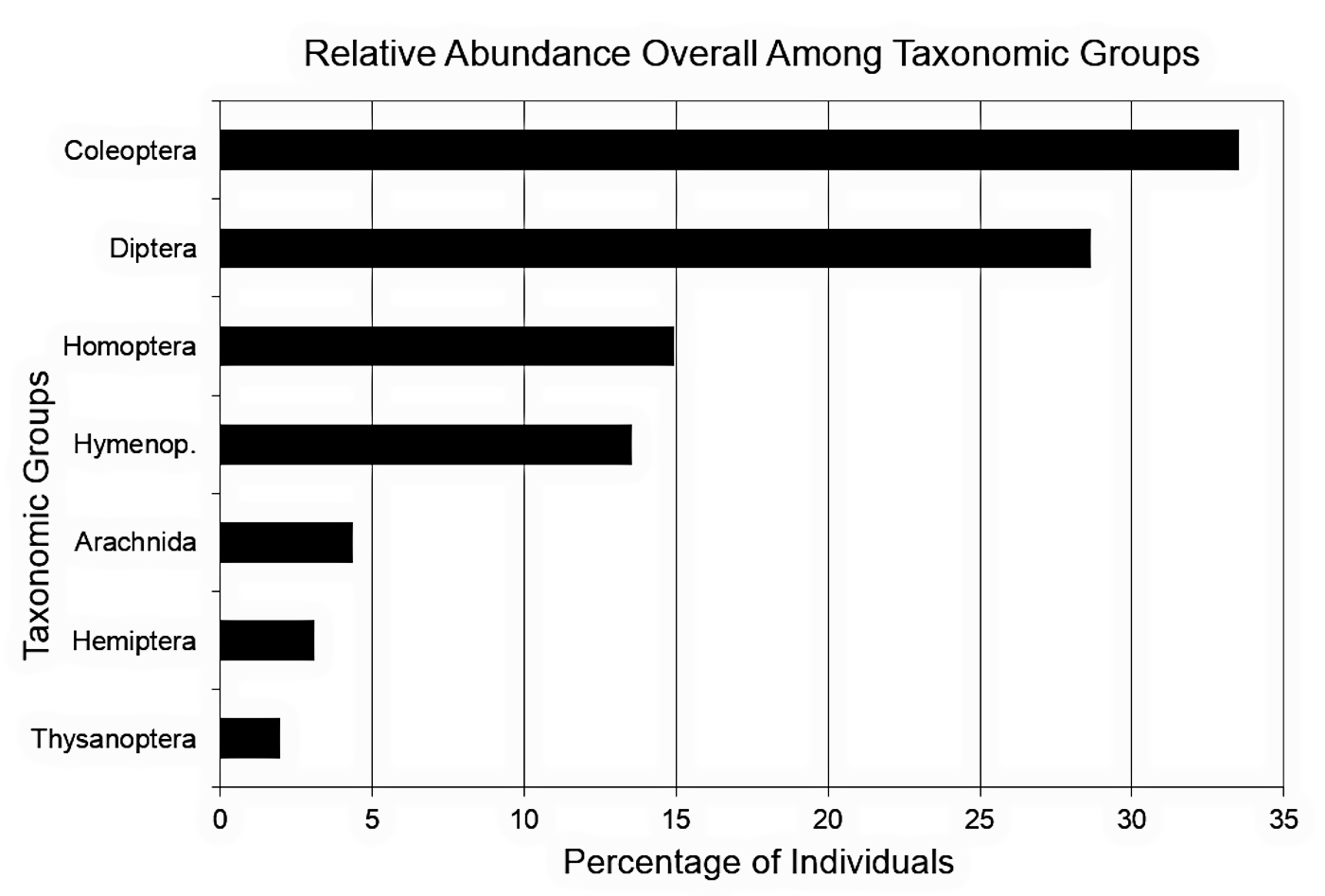
As expected, a one-way χ2 test verified that the captured individuals were not evenly distributed among the different taxonomic groups (figure 1, IV Levels = 7 taxonomic groups, Coleoptera n = 240, Diptera n = 205, Homoptera n = 107, Hymenoptera n = 97, Arachnida n = 31, Hemiptera n = 22, Thysanoptera n = 14, N = 716, df = 6, χ2 = 477.95, p = 0.00). Of the seven taxonomic groups identified, the insect orders Coleoptera (beetles) and Diptera (true flies) dominated, accounting for 62.15% of all the individuals collected.
Figure 1. Relative abundance overall among taxonomic groups. N = 716.
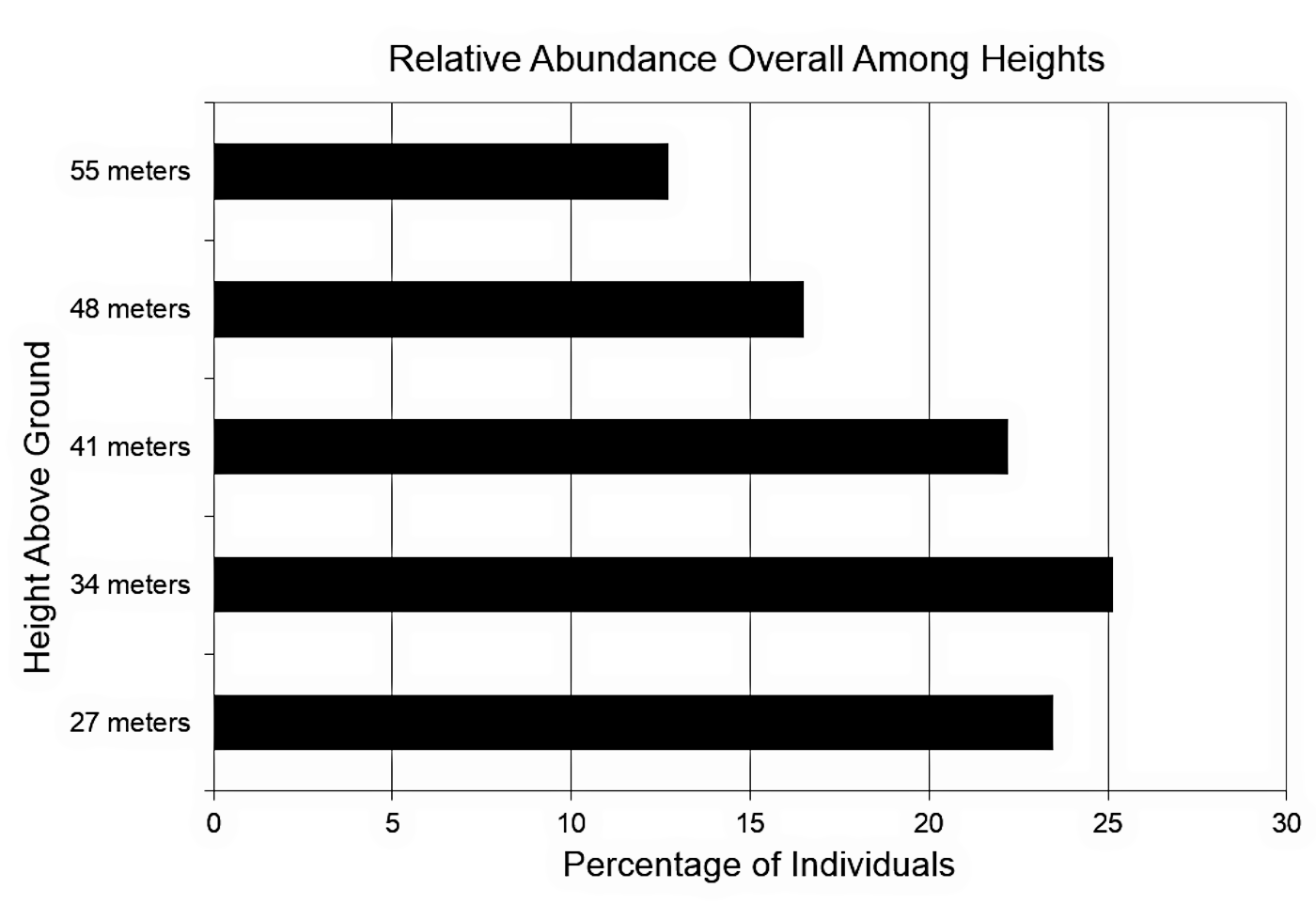
Captured individuals were not evenly distributed among the different heights of the collecting panels (figure 2, one-way χ2 test, IV Levels = 5 heights, 27 m n = 168, 34 m n = 180, 41 m n = 159, 48 m n = 118, 55 m n = 91, N = 716, df = 4, χ2 = 38.96, p = 0.00). The collecting panel at 34 m above the ground captured the greatest number of individuals (25.14%), while the fewest individuals (12.71%) were captured on the highest panel 55 m above the ground.
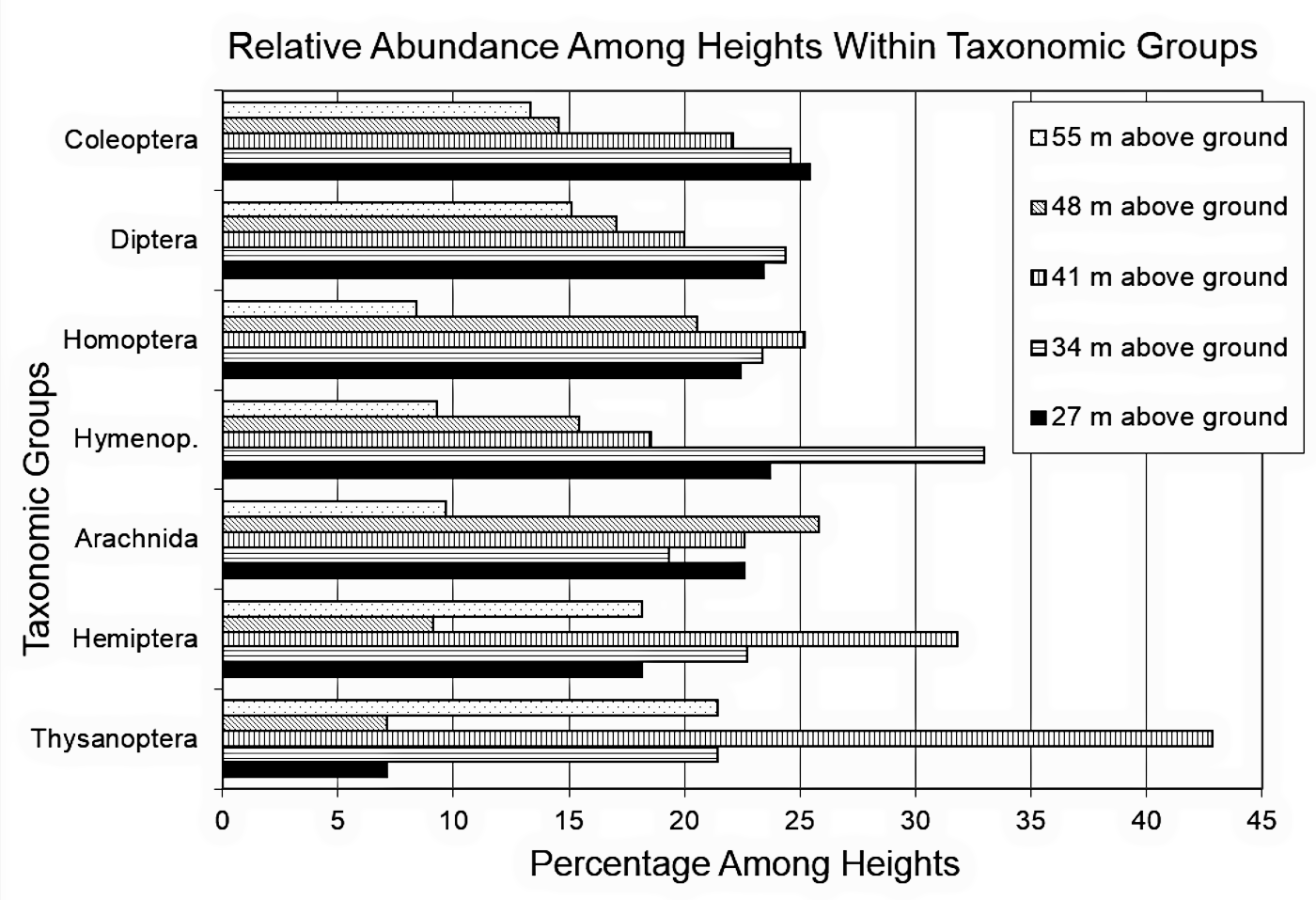
Although some variation was observed in the capture patterns among capture heights within the different taxonomic groups in figure 3, a χ2 test of independence of height above ground and taxonomic group showed no significant difference in the patterns overall (IV1 Levels = 5 heights, IV2 Levels = 7 taxonomic groups, 27 m n = 168, 34 m n = 180, 41 m n = 159, 48 m n = 118, 55 m n = 91, Coleoptera n = 240, Diptera n = 205, Homoptera n = 107, Hymenoptera n = 97, Arachnida n = 31, Hemiptera n = 22, Thysanoptera n = 14, N = 716, df = 24, χ2 = 20.37, p = 0.68); that is, there was no significant difference among heights with respect to taxonomic groups.
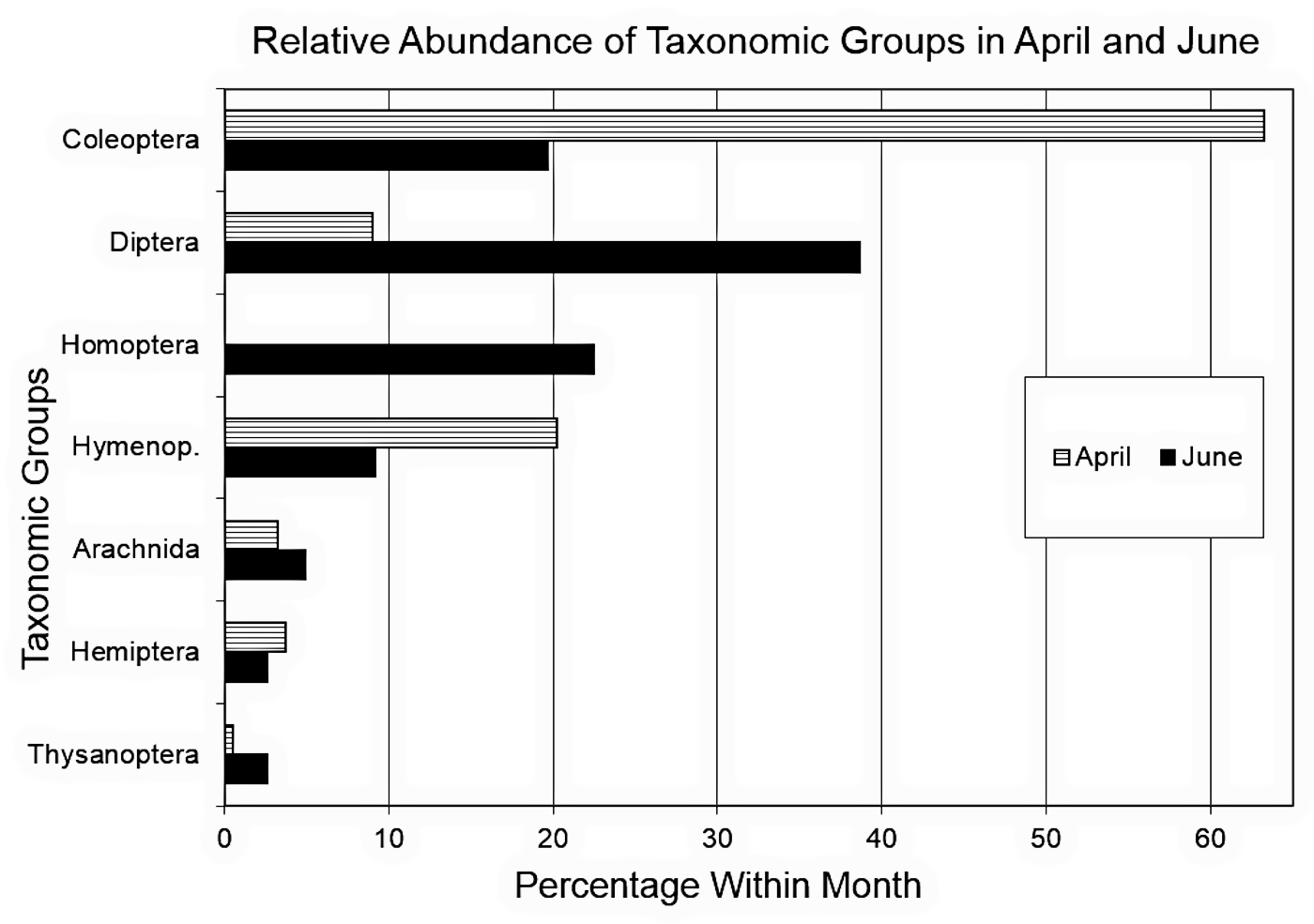
The relative abundance of the taxonomic groups within a month changed dramatically from April to June (figure 4). A χ2 test of independence of taxonomic group and captures in the months of April and June showed a significant difference in the patterns overall (IV1 Levels = 7 taxonomic groups, IV2 Levels = 2 months, Coleoptera n = 211, Diptera n = 198, Homoptera n = 105, Hymenoptera n = 81, Arachnida n = 29, Hemiptera n = 19, Thysanoptera n = 13, April n = 188, June n = 468, df = 6, χ2 = 178.13, p = 0.00); in other words, there was a significant difference among taxonomic groups with respect to month. Especially note the relative abundance decrease in coleopterids and the relative abundance increase in dipterids from April to June. Additionally, in April, homopterids were not present at all, and the relative abundance of hymenopterids decreased noticeably from April to June.
Click for larger image.
Figure 2. Relative abundance
overall among heights. N
= 716.
Click for larger image.
Figure 3. Relative abundance
among heights within taxonomic groups. Coleoptera n
= 240, Diptera n
= 205, Homoptera n
= 107, Hymenoptera n
= 97, Arachnida n
= 31, Hemiptera n
= 22, Thysanoptera n
= 14.
Click for larger image.
Figure 4. Relative abundance
of taxonomic groups in April and June. April n
= 188, June n
= 468.
Furthermore, separately from the perspective of relative abundance, one-way χ2 comparisons of each taxonomic group separately from April to June (IV Levels = 2 months) showed that there were significant differences in the number of individuals captured between months for Diptera (n = 198, df = 1, χ2 = 135.84, p = 0.00), Homoptera (n = 105, df = 1, χ2 = 105.00, p = 0.00), Arachnida (n = 29, df = 1, χ2 = 9.97, p = 0.00), and Thysanoptera (n = 13, df = 1, χ2= 9.31, p = 0.00), but not for Coleoptera (n = 211, df = 1, χ2 = 3.45, p = 0.06), Hymenoptera (n = 81, df = 1, χ2 = 0.31, p = 0.58), and Hemiptera (n = 19, df = 1, χ2 = 1.32, p = 0.25).
DISCUSSION
This aeroecological study presents a snapshot of the relative abundance of aerial insects available for chimney swifts to eat in the town of Indiana, PA, in April, May, and June of 2006 from 27 to 55 m above the ground, where chimney swifts were often seen flying and capturing aerial insects.
The orders of aerial insects captured in this study coincide with the orders of insects that chimney swifts actually eat (Warren 1890, p. 183; Fischer 1958; Kyle and Kyle 2005). However, this is within the context that Hespenheide (1975) determined through gut content analysis that tropical swifts and swallows were very selective (proportionally), relative to what was available.
Assessment of Methodology
Part of this study was to test the feasibility of capturing aerial insects on sticky panels attached to the tether of a helium balloon. The use of a balloon to catch insects in a more urban setting was unconventional, and this study was begun not knowing what to expect relative to success or possible difficulties. The use of acrylic sticky panels in this study was also unconventional, especially in comparison to the more conventional use of nets or other materials to capture aerial insects.
The balloon method was continually hampered by daily weather-related complications and restrictions, and this was its most serious disadvantage. Slightly increased wind speed proved to be unsafe for both researchers and the balloon. On the first test flight before any data were collected, in what was perceived as just a little wind, the balloon forcefully pulled the researcher toward the edge of the roof. Furthermore, the first test flight inflicted major damage to the balloon in the form of several rips; however, since that damage was repaired successfully, the project was able to continue.
Therefore, great care must be taken using this method, and the balloon was only deployed to collect insects on relatively clear days with very little wind and no rain. Experience continued to confirm that constant vigilance was necessary when the balloon was flying. Realistically, the loss of data because of weather restrictions probably had little impact on evaluating what insects were available for chimney swifts. Namely, the change in the feeding behavior of aerial insectivores due to changes in wind, precipitation, temperature, and air density coincides with decreases in aerial insect activity (Kunz et al. 2008; Cox et al. 2019).
As outlined in the Materials and Methods section, this research was very labor-intensive. For example, successfully and efficiently putting up and taking down the balloon required more than one person. A winch mechanism was originally constructed for deploying and retrieving the tethered balloon; however, the attachment mechanisms on the tether rope for the collecting panels were too bulky for the winch to work. The successful solution was to wrap and unwrap the tether rope around the legs of a padded, six-foot-long picnic bench as the balloon was lowered and raised. After the panels were removed from the tether, the process of carefully removing the captured insects from the sticky panels and placing them in jars for later identification was also time-consuming. Removing insects from sticky traps is also a common step in other collecting methods.
As indicated in the next section, other methods, such as two suction-trap methods, use electricity as part of the methodology to catch aerial insects. However, when the balloon method is used, no electricity is needed to capture the insects, which allows the balloon method to be used where electricity is unavailable, as in remote areas away from an urban setting.
This method was successful at capturing insects from a measurable surface area and can be used for relative abundance estimates for multiyear studies. The sticky panels seem to be unbiased in capturing small airborne invertebrates.
Previous Methods of Sampling Aerial Insects
In an early effort to measure the density of flying insects, nets were attached “to the front of a motor car” (Bonnet, A. 1911, as cited in Hardy and Milne 1938). Several researchers in 1933, 1934, and 1935 even tried to collect aerial insects with screens and nets on airplanes, but with little success, collecting only a total of 91 insects in 35 flights, about 2.6 insects per flight (Collins, C. and W. Baker 1934; Berland, L. 1935 as cited in Hardy and Milne 1938). In 1957, Glick (1960) caught 2528 insects in 52 flights using two nets stretched out from either side of a small plane. However, a third of the captured insects were unidentifiable. Other creative ways to capture and track aerial insects have been devised during the last century, including nets on railway trains to track agricultural pests (Hardy and Milne 1938), nets on shortwave radio towers (Freeman 1945), and a combination of nets on kites and radar tracking (Chen et al. 1989).
Hardy and Milne (1938) successfully used kites to place collecting nets at various heights above the ground, ranging from 150 ft (45.72 m) to almost 2000 ft (609.6 m) above the ground. They collected 839 individuals (including one spider) during 124.5 h over four summers in Hull, England, in comparison to this study in Pennsylvania, in which 716 individuals were captured in 115.84 h over three months in the transition from spring to summer. The overall capture rate using the kites and nets was 6.74 captures per hour, while the overall capture rate for this study was 6.18 captures per hour (recognizing that factors other than technique would influence these values). Comparing only similar sampling heights from the Hardy and Milne (1938) study and this study (150, 175, and 200 ft: 45.72 m to 60.96 m above the ground) yielded one result of relative abundance among taxonomic groups similar to ours. Namely, the order Diptera had the highest relative abundance (53.9%) in the summer in England, while Diptera also had the highest relative abundance (38.7%) in Pennsylvania in June (but not earlier in April; figure 4). However, the other orders in that study ranked very differently compared to this study. Just as in this study, their efforts were very labor-intensive and weather-dependent.
In England and Scotland, a suction-trap network consisting of sixteen stations continues to be hosted by Rothamsted Research and funded by the United Kingdom’s Biotechnology and Biological Sciences Research Council to catch migrating aphids (Rothamsted Research 2020). Muirhead-Thomson (1991, 66–93) explained that each sampling station consists of a 12.2-m-high tower that was developed to collect migrating aphids to provide “a better understanding of aphid biology in relation to transmission of plant virus diseases . . . an aphid warning system.” The tower structure contains “an electric fan and the necessary filter and storage devices to collect the insect sample. The trap inlet is 12.2 m above ground.” A similar multistate U.S. Soybean aphid suction trap network is also in operation, with trap intakes at 5.8 m above the ground (Lagos-Kutz et al. 2020). This method has also been used to successfully study insects available to nesting tree swallows (Tachycineta bicolor; McCarty and Winkler 1999).
Other researchers before the data collection of this study have used balloons to sample migrating aerial insects (Riley et al. 1995; Chapman et al. 2004). However, those studies were done at higher altitudes than this study and used collecting nets rather than sticky panels. After the data collection of this study, the Malaria Research and Training Center of Mali conducted a study of the windborne long-distance, high-altitude migration of malaria mosquitoes in the Sahel of Africa (Huestis et al. 2019). Rectangular, sticky “panels,” made from tulle netting, covered with the same adhesive as in this study, and attached to the balloon’s tether with Velcro, were used to sample mosquitoes up to 290 m above the ground. The collecting panels functioned the same way as in this study, and they also reported many practical difficulties involving wind and equipment damage using the balloon method. The authors of the Huestis et al. (2019) study acknowledged that their balloon method was adapted from an earlier study on high-altitude mating flights in fire ants in Florida by Fritz, Fritz, and Vander Meer (2011).
In contrast to the studies above using a balloon, this study used a balloon to investigate the local populations of aerial insects at lower heights using acrylic sticky panels.
Recommendations for Future Researchers
With our experience with the balloon method and its inherent problems, two recommendations for future researchers are the following.
An approach to consider is to erect long poles vertically from the roofs of buildings along with attached acrylic sticky collecting panels. Although the balloon method is less expensive than more elaborate approaches, the rooftop-pole method would be an even less expensive approach. Although we have not directly tested this idea ourselves, small sticky traps, such as Petri dishes, have been placed and used successfully on 1.5–7.0 m vertical poles in the ground in the past (Poland, McCullough, and Anulewicz 2011; Smith, Kennedy, and Muehlbauer 2014; Tabuchi et al. 2017). An extension of this method at greater heights could work exceptionally well in urban environments where chimney swifts are likely to be present. Using many collection stations throughout a city, with tall poles attached to rooftops at varying heights, would result in less dependence on weather, many more sampling heights, increased collection time, and ease of maintenance. Having mechanisms near the base of the poles to raise and lower sticky collecting panels with a pulley system, or to fold down the poles horizontally for attaching and detaching panels, is recommended.
We also recommend using the suction-trap method, as described above by Rothamsted Research (2020) and Muirhead-Thomson (1991, 66–93), on urban rooftops. However, this would be a more elaborate and expensive method.
CONCLUSIONS
In summary, the balloon method described in this study was successful in collecting aerial insects available for chimney swifts to eat above Indiana, PA. We conclude that this study can provide a useful baseline dataset for future studies on the relative abundance of aerial insects in western Pennsylvania. Although the data retrieved using this method are useful, we also conclude that the method is problematic with practical difficulties. We recommend that future aerial insect sampling be done by either the rooftop-pole method described above, or the more expensive suction-trap method also described above.
ACKNOWLEDGMENTS
This research was partially supported by a $1500 grant from the Senate Research Committee Small Grants Program of Indiana University of Pennsylvania to cover the cost of such items as the balloon and a reliable supply of helium, and that support is gratefully acknowledged. This project would not have been possible without assistance, and we gratefully acknowledge more than 30 people who helped with a variety of tasks, such as taking the balloon down safely at the end of a sampling period. The authors also thank the anonymous reviewers for their essential contributions.
WORKS CITED
Adler, J. 2020. Wild man. Smithsonian 51(1): 80–94.
Berland, L. 1935. Premier résultats de mes recherches en avion sur la faune et la flore atmosphériques. Ann. Soc. Ent. Fr. 104: 73–96, quoted in A. Hardy and P. Milne. 1938. Studies in the distribution of insects by aerial currents: Experiments in aerial tow-netting from kites. J. Anim. Ecol. 7(2): 199–229.
BirdLife International. 2018. Chaetura pelagica. The IUCN Red List of Threatened Species 2018: e.T22686709A131792415. Available HERE.
Bonnet, A. 1911. Recherches sur les causes des variations de la faunule entomologique aérienne. C.R. Acad. Sci. Paris 152: 336–39, quoted in A. Hardy and P. Milne. 1938. Studies in the distribution of insects by aerial currents: Experiments in aerial tow-netting from kites. J. Anim. Ecol. 7(2): 199–229.
Brauning, D., ed. 1992. Atlas of Breeding Birds in Pennsylvania. Pittsburgh, PA: University of Pittsburgh Press.
Chapman, J., D. Reynolds, A. Smith, E. Smith, and I. Woiwod. 2004. An aerial netting study of insects migrating at high altitude over England. Bull. Entomol. Res. 94: 123–36.
Chen, R., X. Bao, V. Drake, R. Farrow, S. Wang, Y. Sun, and B. Zhai. 1989. Radar observations of the spring migration into northeastern China of the oriental armyworm moth, Mythimna separata, and other insects. Ecol. Entomol. 14(2): 149–62.
Collins, C. and W. Baker. 1934. Exploring the upper air for wind-borne gipsy moth larvae. J. Econ. Entomol. 27: 320–23, quoted in A. Hardy and P. Milne. 1938. Studies in the distribution of insects by aerial currents: Experiments in aerial tow-netting from kites. J. Anim. Ecol. 7(2): 199–229.
COSEWIC. 2007. COSEWIC assessment and status report on the chimney swift Chaetura pelagica in Canada. Species at Risk Public Registry, Committee on the Status of Endangered Wildlife in Canada, Ottawa. Available HERE.
COSEWIC. 2018. COSEWIC assessment and status report on the chimney swift Chaetura pelagica in Canada. Species at Risk Public Registry, Committee on the Status of Endangered Wildlife in Canada, Ottawa. Available HERE
Cox, A., R. Robertson, A. Lendvai, K. Everitt, and F. Bonier. 2019. Rainy springs linked to poor nestling growth in a declining avian aerial insectivore (Tachycineta bicolor). Proc. Roy. Soc. B Biol. Sci. 286(1898): 20190018.
Ferro, M. 2011. Collecting Insects. Center for Invasive Species and Ecosystem Health, University of Georgia. Available HERE.
Fischer, R. 1958. The Breeding Biology of the Chimney Swift Chaetura pelagica (Linnaeus). New York State Museum and Science Service Bulletin Number 368. The University of the State of New York, Albany.
Fitzgerald, T., E. van Stam, J. Nocera, and D. Badzinski. 2014. Loss of nesting sites is not a primary factor limiting northern chimney swift populations. Pop. Ecol. 56: 507–12.
Freeman, J. 1945. Studies in the distribution of insects by aerial currents. J. Anim. Ecol. 14(2): 128–54.
FreeMapTools. 2020. Free Map Tools. HERE
Fritz, G., A. Fritz, and R. Vander Meer. 2011. Sampling high-altitude and stratified mating flights of red imported fire ant. J. Med. Entomol. 47(3): 508–12.
Glick, P. 1960. Collecting Insects by Airplane, with Special Reference to Dispersal of the Potato Leafhopper. Technical Bulletin No. 1222. U.S. Department of Agriculture, Washington, DC.
Hallmann, C., M. Sorg, E. Jongejans, H. Siepel, N. Hofland, H. Schwan, W. Stenmans, A. Müller, H. Sumser, T. Hörren, D. Goulson, and H. de Kroon. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12(10): e0185809.
Hardy, A., and P. Milne. 1938. Studies in the distribution of insects by aerial currents: Experiments in aerial tow-netting from kites. J. Anim. Ecol. 7(2): 199–229.
Hespenheide, H. 1975. Selective predation by two swifts and a swallow in Central America. Ibis 117(1): 82–99.
Huestis, D., A. Dao, M. Diallo, Z. Sanogo, D. Samake, A. Yaro, Y. Ousman, Y. Linton, A. Krishna, L. Veru, B. Krajacich, R. Faiman, J. Florio, J. Chapman, D. Reynolds, D. Weetman, 2019. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature 574: 404–8.
Kunz, T., S. Gauthreaux, Jr., N. Hristov, J. Horn, G. Jones, E. Kalko, R. Larkin, G. McCracken, S. Swartz, R. Srygley, R. Dudley, J. Westbrook, and M. Wikelski. 2008. Aeroecology: Probing and modeling the aerosphere. Integr. Comp. Biol. 48(1): 1–11.
Kyle, P., and G. Kyle. 2005. Chimney Swifts: America’s Mysterious Birds above the Fireplace. College Station, TX: Texas A&M University Press.
Lagos-Kutz, D., D. Voegtlin, D. Onstad, D. Hogg, D. Ragsdale, K. Tilmon, E. Hodgson, C. Difonzo, R. Groves, C. Krupke, J. Laforest, N. Seiter, E. Duerr, B. Bradford, and G. Hartman. 2020. The soybean aphid suction trap network: Sampling the aerobiological “soup.” Am. Entomol. 66(1): 48–55.
McCarty, J., and D. Winkler. 1999. Foraging ecology and diet selectivity of tree swallows feeding nestlings. Condor 101(2): 246–54.
Michel, N., A. Smith, R. Clark, C. Morrissey, and K. Hobson. 2016. Differences in spatial synchrony and interspecific concordance inform guild-level population trends for aerial insectivorous birds. Ecography 39(8): 774–86.
Muirhead-Thomson, R. 1991. Trap Responses of Flying Insects. London: Academic Press.
Nebel, S., A. Mills, J. McCracken, and P. Taylor. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conserv. Ecol. 5(2): Article 1.
Nocera, J., J. Blais, D. Beresford, L. Finity, C. Grooms, L. Kimpe, K. Kyser, N. Michelutti, M. Reudink, and J. Smol. 2012. Historical pesticide applications coincided with an altered diet of aerially foraging insectivorous chimney swifts.
Proc. Biol. Sci. 279(1740): 3114–20.
Pennsylvania Game Commission and Pennsylvania Fish and Boat Commission. 2015. Pennsylvania Wildlife Action Plan, 2015–2025, C. Haffner and D. Day, eds. Harrisburg, PA: Pennsylvania Game Commission and Pennsylvania Fish and Boat Commission. Available HERE.
Poland, T., D. McCullough, and A. Anulewicz. 2011. Evaluation of double-decker traps for emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 104(2): 517–31.
Riley, J., D. Reynolds, A. Smith, A. Edwards, X. Zhang, X. Cheng, H. Wang, J. Cheng, and B. Zhai. 1995. Observations of the autumn migration of the rice leaf roller Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) and other moths in eastern China. Bull. Entomol. Res. 85: 397–414.
Rothamsted Research. 2020. The Insect Survey. Available HERE.
Sauer, J., D. Niven, J. Hines, D. Ziolkowski, Jr., K. Pardieck, J. Fallon, and W. Link. 2017. The North American Breeding Bird Survey, Results and Analysis 1966–2015, Version 2.07.2017. USGS Patuxent Wildlife Research Center, Laurel, MD. Available HERE.
Smith, J., T. Kennedy, and J. Muehlbauer. 2014. Building a better sticky trap: Description of an easy-to-use trap and pole mount for quantifying the abundance of adult aquatic insects. Freshwater Sci. 33(3): 972–77.
Tabuchi, K., A. Takahashi, N. Hinomoto, and M. Shimoda. 2017. Potential enhancement of the number of trap captures of minute pirate bugs (Orius spp.) on blue sticky traps: The effect of visual contrast. Ann. Report Plant Protect. N. Japan 68: 168–72.
Tallamy, D., and W. Shriver. 2021. Are declines in insects and insectivorous birds related? Ornithological Applications 123(1): duaa059.
Todd, W. 1940. Birds of Western Pennsylvania. Pittsburgh, PA: University of Pittsburgh Press.
Wagner, D., E. Grames, M. Forister, M. Berenbaum, and D. Stopak. 2021. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. 118(2): e2023989118.
Warren, B. 1890. Report on the Birds of Pennsylvania. With Special Reference to the Food-Habits, Based on over Four Thousand Stomach Examinations, 2nd ed. Harrisburg, PA: E. K. Myers, State Printer.
Wilson, A., D. Brauning, and R. Mulvihill, editors. 2012. Second Atlas of Breeding Birds in Pennsylvania. University Park: Pennsylvania State University Press.
Zammuto, R., E. Franks, and C. Preston. 1981. Factors associated with the interval between feeding visits in brood-rearing chimney swifts. J. Field Ornithol. 52(2): 134–39.
| Front Page |
Dr. Winstead's Blood Pressure Tracker: Free Templates for Graphing Blood Pressure in Microsoft Excel
Dr. Winstead's Current Local and World Standard Percentage Metric Time Clock